Crustose coralline algae and sedimentation
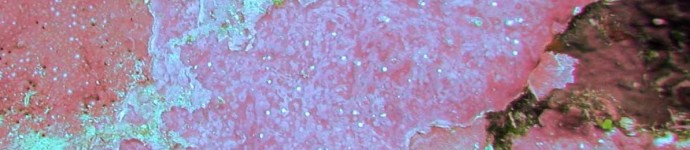
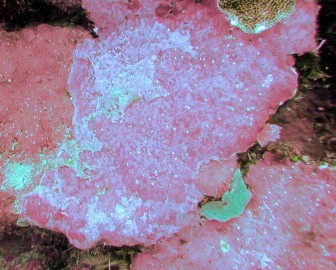
Crustose coralline algae (CCA) are rock-hard calcareous red algae that fulfill two key functional roles in coral reef ecosystems: they contribute significantly to reef calcification and cementation, and they induce larval settlement of many benthic organisms.
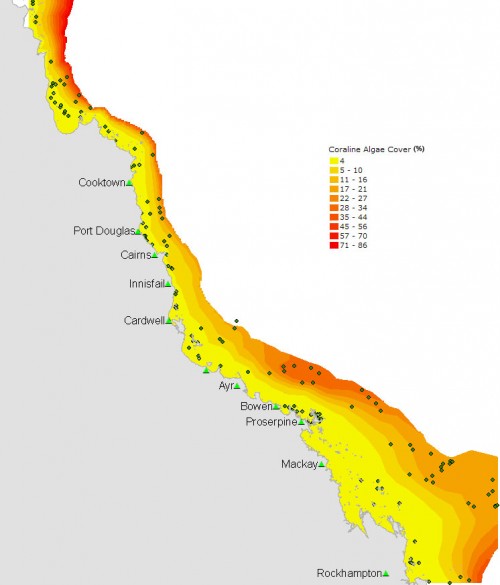
A study of CCA cover on the Great Barrier Reef (GBR) shows strong differences in cover across the Continental Shelf. Mean CCA cover is <1% on the inner third of the GBR, compared to >20% on the outer half of the shelf. CCA cover is also affected by the amount of sediment deposited, water clarity, and the steepness of the reef slopes. Within each cross-shelf zone, the cover of CCA is higher on reefs with low sediment deposits than on reefs with high levels of sedimentation. On the inner third of the shelf, the most sediment-exposed reefs are unsuitable habitats for most CCA. This inverse relationship between CCA and sediment has implications for the recruitment of CCA-specialised organisms, and for the balance between reef accretion and erosion.
Details of this study are published in Fabricius and De'ath (2001).
Introduction
Crustose coralline algae (CCA) are rock-hard calcareous red algae that serve two key functions in coral reefs. Their calcified encrustations reinforce dead coral skeletons and fill cracks in the reef substratum, contributing to reef formation and cementation, maintaining a complex reef surface and reducing reef erosion. The larvae of many reef organisms, including hard corals, favour CCA as a substrate for growing on.
Methods
To investigate the main spatial and environmental factors that determine CCA cover on the GBR, the researchers surveyed 144 reefs between latitude 10 to 24 degrees South (Fig. 1; Fabricius 2001). Observers recorded the following data at five depth ranges:
- Visual estimates of percent cover of CCA, hard corals, octocorals, turf algae, Sargassum and other macroalgae, and unconsolidated substratum (sand/sediment/mud; and rubble)
- Thickness of sediment deposit on the reef substratum (rated on a 4-point scale; 0 = none, 1 = thin layer, 2 = moderate layer, and 3 = thick deep layer)
- Visibility (Secchi distance; m)
- Slope angle.
Results: What determines CCA cover on the GBR?
The surveys show that CCA cover, sediment, visibility, and slope change systematically across, but not along, the GBR. CCA averages <1% cover on the inner-shelf and ~20% on the outer-shelf (Fig. 2). As well as this strong change across the shelf, CCA cover is also related to sediment, visibility and slope angle (Fig. 3).
CCA cover is highest at low sediment deposits, high visibility and steep slope angles. Sediment deposits are lower on steep slopes than on gradual slopes and visibility decreases with increasing levels of sediment. A statistical analysis of the results showed three distinct regions of CCA communities across the shelf (inshore, a narrow inner- to mid-shelf band and mid-shelf and offshore). In each of these regions, higher levels of sediment are related to lower levels of CCA. On the inner shelf, all reefs with high sediment had <0.5% CCA cover. The analyses explain variation in CCA cover very well (80% of variation is explained).
Discussion
The analyses suggest that CCA cover on reefs of the Great Barrier Reef is strongly related to distance across the shelf and sedimentary deposits on the reefs, and weakly related to water clarity (visibility) and the steepness of reef slopes. Within each of the three CCA regions identified, cover depends on sedimentation. The consistency of these three independent correlations perhaps suggest a causal relationship between sedimentation and CCA cover. However, since causality cannot be established from observations, controlled experiments will be necessary to identify whether and how sediments ultimately affect CCA life histories.
The absence of CCA under sediment was confirmed numerous times, by fanning off the sediment and visually inspecting the substratum below. CCA crusts were found only occasionally underneath thin to moderate sediment deposits, and patches were generally small (up to a few cm2). By contrast, turf algae that compete with CCA for space were regular features under thin to moderate sediments, apparently holding the sediment in place. Very little turf and crustose coralline algae were found underneath thick sediment deposits.
The mechanisms of how sediment deposits may affect CCA are unknown. CCA can survive long periods of burial but sediment does appear to reduce their abundance. Even though it may not kill them, it is likely that sediment reduces CCA photosynthesis by shading and restriction of gas exchange (Patterson 1991), thus reducing scope for growth, and therefore contribution to reef calcification. Sediments also blanket substrate for settlement of CCA and they effectively remove CCA as settlement substrate for other organisms during times of burial. Hence, CCA are unable to fulfil their two key functional roles in coral reefs (calcification and settlement substrate) whilst buried under sediment.
Little sediment accumulates on reefs in a geological sense, as reef topography facilitates sediment transport downslope onto the sea floor. Nevertheless, thick sediment deposits are regularly encountered on coastal reefs, with highest values found on the lower leeward sides of reefs (Wolanski 2005). These sediment deposits are only shifted during cyclonic events.
Experiments have shown that sediments containing traces of the herbicide diuron kill CCA far more quickly than sediments free of herbicides (Harrington 2005). This indicates that the disruption of photosynthesis under the sediment layer causes irreversible damage, possibly accelerating the smothering effects of sedimentation. Other studies have shown that organically rich sediments cause more damage to corals than clean sediments (Weber 2007). While the potential effects of sedimentation of such nutrient-enriched particles on CCA are as yet unknown, they deserve attention as the import of sediments and nutrients into the GBR lagoon during run-off events has increased several-fold due to expanding grazing and agricultural land use (Furnas 2003; McKergow 2005).
The implications of low CCA cover in coastal areas with high sedimentation on the ecology of near-shore coral reefs are unknown. Caves and cavities below the surface, which are inhabited by diverse and trophically important communities, and organisms depending on surface complexity for protection, disappear when the reef structure collapses. Organisms specialised in settling on CCA are also excluded from areas which are unsuitable for the growth of CCA. The absence of CCA on sediment-rich near-shore reefs may partly explain why community composition and species abundances on reefs close to the coast are so different from those further offshore.
Further Reading
De'ath G, Fabricius KE (2000) Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 81:3178-3192
Fabricius K, De'ath G (2001) Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 19:303-309
Furnas MJ (2003) Catchments and Corals: Terrestrial Runoff to the Great Barrier Reef. Australian Institute of Marine Science, CRC Reef.
Harrington L, Fabricius K, De'ath G, Negri A (2004) Fine-tuned recognition and selection of settlement substrata determines post-settlement survival in corals. Ecology 85:3428-3437
Harrington L, Fabricius K, Eaglesham G, Negri A (2005) Synergistic effects of diuron and sedimentation on photosynthetic yields and survival of crustose coralline algae. Marine Pollution Bulletin 51:415-427
McKergow L, Prosser I, Hughes A, Brodie J (2005) Sources of sediment to the Great Barrier Reef World Heritage Area. Marine Pollution Bulletin 51:200-211
Patterson MR, Sebens KP, Olson RR (1991) In situ measurements of flow effects on primary production and dark respiration in reef corals. Limnology and Oceanography 36:936-948
Therneau T (1998) RPART software from statlib@lib.statlib.cmu.edu.
Weber M, Lott C, Fabricius KE (2007) Sedimentation stress in a scleractinian coral exposed to terrestrial and marine sediments with contrasting physical, organic and geochemical properties. Journal of Experimental Marine Biology and Ecology 341:151-151
Wolanski E, Fabricius K, Spagnol S, Brinkman R (2005) Fine sediment budget on an inner-shelf coral-fringed island, Great Barrier Reef of AustraliaEstuarine and Coastal Shelf Science:153-158