Toxicology testing of herbicides entering the Great Barrier Reef

Pesticides from agricultural sources have been detected throughout the year in the Great Barrier Reef World Heritage Area (RRMMP). Australia has strict standards and monitoring to limit the amount of pesticides allowed in food products. However, the fate and impact on the environment of toxic chemicals applied to crops is less well known. For example, the effects on coastal marine organisms from the concentrations of herbicides found in GBR coastal waters is under debate and is the subject of several research projects.
As research into the effects of pesticides on GBR organisms accumulates, the standards, definitions and methods of measurement are being refined. The risk of exposing lifeforms in GBRWHA to herbicides is assessed from the results of toxicology testing. Toxicology is the study of the adverse effects of chemicals (or sometimes physical) agents on living organisms and involves a four step process, the final step of which is risk characterisation.
- Hazard Identification
- Dose Response Assessment
- Exposure Assessment
- Risk Characterisation
Hazard Identification
The book ‘Silent Spring’ by Rachel Carson published in 1962 was a milestone in broadening public awareness that the chemicals used to control pests in agricultural systems can have detrimental effects on humans and the environment. Carson’s book described how chemicals such as DDT were killing wildlife and how pesticides and their breakdown products are accumulated up the food chain.
DDT was banned from use in Australia in 1987 but new compounds and chemical variants are continually being developed to increase crop productivity in Australian agriculture. The group of weed killers known as PSII inhibiting herbicides are the most commonly detected group of chemical toxins from land-based sources identified as hazards to organisms in waters of the GBR. PSII herbicides are classified by the mode of action that kills the weed; in this case photosynthetic processes are blocked causing cell death. The target molecule for PSII herbicides is very similar for most plants, meaning that the herbicide is equally toxic to target (weeds) and non-target organisms (seagrass and corals) that are exposed as a result of runoff from agricultural sources.
Dose Response Assessment
Guideline values that define the “safe” or acceptable level for a particular toxin in the environment are calculated from dose – response experiments. The Australian and New Zealand Water Quality Guidelines are moving towards the international best practice in methods to derive guideline values for toxicity and GBRMPA has derived its own guideline values for relevant toxicants and contaminants 1. Guideline (or trigger) values are derived by ranking the sensitivity of several species (more is better) and then calculating the toxicant concentrations that affect different proportions of species. The conservation values of the GBR are so high that GBRMPA recommends that 99% species protection guidelines are applied, which means toxicant concentrations should remain below levels that affect 1% of relevant species 1.
The current Australian guideline for diuron is 0.2 µg per litre2 while the GBRMPA guideline for 99% species protection is currently 0.9 µg per litre 1. New national and GBRMPA-specific guidelines are likely to be updated in 2015 based on recent toxicological studies.
Understanding the sensitivity of seagrass and corals to herbicides involves exposing these species to the herbicides at different concentrations in highly controlled experimental setups and accurately measuring their response. As the dose increases so does the effect and this is plotted as a dose-response curve, an example of which is shown for seasgrass exposure to the herbicides Diuron, Atrazine, Hexazinone and Tebuthiuron (Figure 1)3.
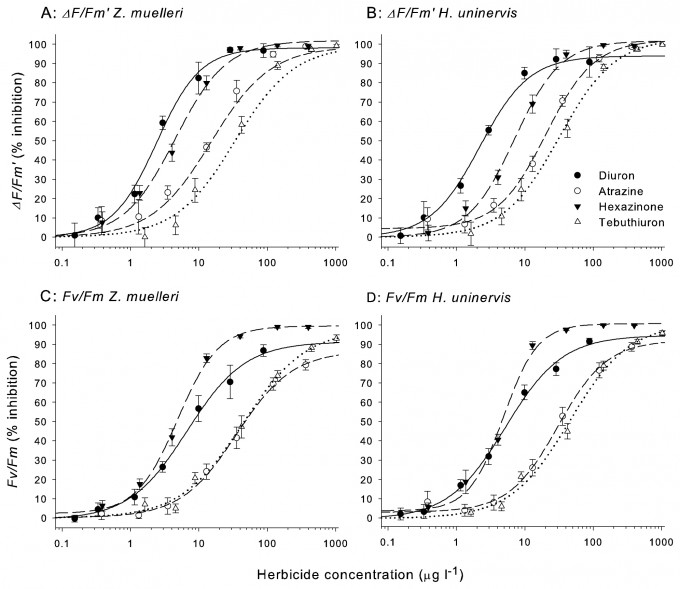
NOEC values indicate the concentration where no measureable response occurs in the test organism. While NOEC values are important for setting guideline values, calculating EC10 and EC50 values from dose-response curves are considered a more reliable measure of effect. These values represent the concentrations of the toxin that cause an effect of 10 and 50% respectively (Figure 2)3. For example, the herbicide Diuron, affects the photosynthesis in seagrasses, corals and microalgae by 10% at a concentration of ~ 0.5 µg per litre which is therefore its EC10 for these species.
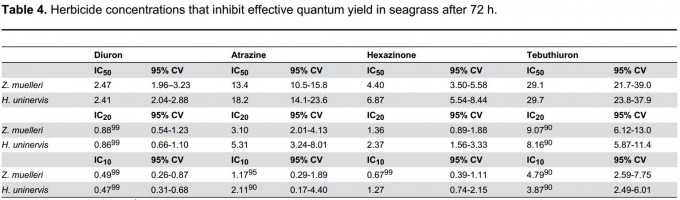
According to Dr Andrew Negri, program leader of NERP project 4.2 more toxicity tests are needed for all the pesticides we detect in the GBR. Tests should include the species of highest conservation value to the marine park such as seagrass and corals3,5,6. He said however that “guideline values derived from laboratory experiments may underestimate risk to the environment as most experiments are conducted under optimal conditions and in the absence of other co-stressors which often occur in nature”.
Guideline values based on dose-response curves may need to be modified to account for the effects of these additional stressors and potential differences in sensitivity of different life stages. It has been repeatedly demonstrated that if corals and other invertebrates are affected by a second stressor (such as high temperatures frequently encountered in the GBR) that they become more sensitive to PSII herbicides5,6. Similarly light levels can interact with toxins. For example, in a flood-plume, seagrass faces low light levels and herbicide exposure at the same time and both of these stressors are likely to reduce photosynthesis and eventually primarily production in the seagrass bed ecosystem7.Multiple PSII herbicides are often detected simultaneously,8 which may increase the risk to coastal ecosystems. Dr Negri said NERP projects have confirmed that, “when more than one PSII herbicide is detected the method of concentration addition (CA) accurately estimates the total toxicity of the water body and this figure can be used to assess risk to GBR habitats.
Exposure Assessment
PSII herbicide concentrations have been detected in flood plumes entering the GBR at concentrations higher than Australian and GBR guideline values4 and at these concentrations would negatively affect phootosysntheis in seagrass and corals 3,5,6 and growth in tropical algae7. The highest concentrations of herbicides are detected close to river mouths but their high mobility in seawater and slow breakdown means that they can be detected far from their source and months later than the original discharge into the GBR lagoon.
Risk Assessment
The Scientific Consensus statement on Land use impacts on Great Barrier Reef water quality and ecosystem condition (2013) found that “in coastal seagrass habitats and freshwater and estuarine wetlands, pesticides can pose a high risk. Concentrations of a range of pesticides exceed water quality guidelines in many fresh and estuarine water bodies downstream of cropping lands. Based on a risk assessment of the six commonly used photosystem II inhibiting herbicides, the Mackay Whitsunday and Burdekin regions are considered to be at highest risk, followed by the Wet Tropics, Fitzroy and Burnett Mary regions. However, the risk of only a fraction of pesticides has been assessed, with only six of the 34 pesticides currently detected included in the assessment, and therefore the effect of pesticides is most likely to have been underestimated.”
1. Water quality guidelines for the Great Barrier Reef Marine Park 2010 [electronic resource] / Great Barrier Reef Marine Park Authority ISBN 978 1 921682 29 2 (pdf).
2. Australian and New Zealand guidelines for fresh and marine water quality. Volume 1, The guidelines / Australian and New Zealand Environment and Conservation Council, Agriculture and Resource Management Council of Australia and New Zealand ISBN 09578245 0 5 (set).
3. Flores F, Collier CJ, Mercurio P, & Negri AP (2013) Phytotoxicity of four photosystem II herbicides to tropical seagrasses. PLoS ONE 8(9):e75798.
4. Lewis SE, et al. (2009) Herbicides: A new threat to the Great Barrier Reef. Environ. Pollut. 157:2470-2484.
5. Negri AP & Hoogenboom MO (2011) Water contamination reduces the tolerance of coral larvae to thermal stress. PLoS ONE 6(5):e19703.
6. Jones RJ & Kerswell AP (2003) Phytotoxicity of photosystem II (PSII) herbicides to coral. Mar. Ecol. Prog. Ser. 261:149-159.
7. Magnusson M, Heimann K, & Negri AP (2008) Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Mar. Pollut. Bull. 56(9):1545-1552.
8. Lewis SE, et al. (2012) Assessing the additive risks of PSII herbicide exposure to the Great Barrier Reef. Mar. Pollut. Bull. 65(4–9):280-291.





