Coral recovery - are reef communities similar before and after disturbance?
Corals are the back bones of coral reef ecosystems. They produce calcium carbonate skeletons that build coral reef framework. Reef fishes and many other inhabitants depend upon corals for shelter and food. Coral populations are dynamic, cycling through phases of disturbance and recovery. Many types of disturbance kill corals including cyclones, Crown-of-Thorns Starfish (COTS) outbreaks, bleaching and disease. Corals can recover from these impacts through the dispersal and recruitment of sexually reproduced larvae and through asexual propagation such as the growth of adult fragments (Baird et al. 2009).
Corals differ in their susceptibility to disturbances and also in their capacity to recover depending on their life history traits (Hughes and Tanner 2000). For example, many Acropora species have fragile skeletons that break easily in cyclonic conditions. They are the also preferred food of COTS (De’ath and Moran 1998). However, Acropora spp. can recover from a disturbance relatively quickly (within 10 years; Halford et al. 2004) through the successful recruitment and rapid growth of dispersed larvae. In contrast, coral in the genus Porites are the least favoured food of COTS and many species have strong growth forms that are more resistant to physical damage by heavy seas. However, these species grow slowly and their simple physical forms cannot afford protection to reef inhabitants in the manner that corals with more complex structures can (Graham et al. 2006).
Recovery of coral communities is typically assessed by measuring changes in coral cover so that a coral reef is deemed recovered once coral cover reaches the pre-disturbance level (Graham et al. 2011). This simple measure overlooks the complexities of coral recovery, and an assessment of recovery based solely on coral cover can be misleading. Changes in the proportional abundances of coral genera and their growth forms (community composition) during recovery can have consequences for ecological processes that are critical to the health of the ecosystem. The community composition can be restored to the pre-disturbance structure so that previous ecosystem processes are reinstated (Nystrom et al. 2008). Alternatively, changes in the community composition during recovery can cause associated shifts in ecological processes, which may be detrimental to the health of the ecosystem (see Aronson et al. 2004). Understanding how the composition of coral communities change during recovery from disturbance and the consequences of these changes is crucial to assessing the health and future of coral reefs.
Scientists from the Australian Institute of Marine Science (AIMS) have used data from the Long-term Monitoring Program (LTMP) to examine the recovery of coral community composition (reassembly) on reefs on the Great Barrier Reef (Johns et al 2014). The six focus reefs all suffered massive coral loss around the turn of the century that was caused by various disturbance types including COTS outbreaks, cyclones and bleaching.
Coral communities all reassembled to some degree as coral cover increased during a period of recovery. However, the rate of reassembly was different depending on the type of community that existed at the reef before the disturbance (Figure 1).
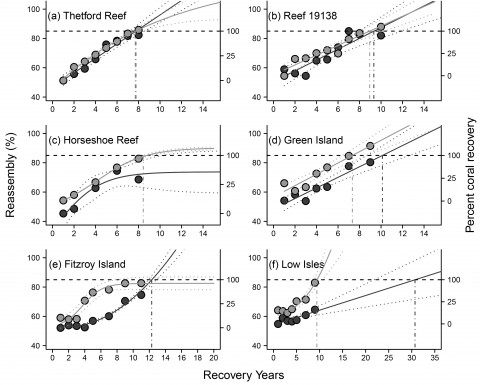
Reefs that had a high abundance of tabulate Acropora spp. recovered their community composition within 7-10 years. This coral group was the most heavily impacted by disturbance and rapid recovery in community composition was driven by the swift recovery of this group. The rate of reassembly for these reefs matched the recovery of coral cover. Reefs that were characterised by a different suite of corals, particularly Porites spp, Montipora spp. and soft corals, recovered coral cover in a similar time frame, but reassembly of the coral community lagged behind. At Low Isles, a fringing reef island north-east of Port Douglas, reassembly of the coral community to the pre-disturbance composition seemed unlikely within 30 years of recovery, though coral cover had returned to pre-disturbance levels within 10 years (Figure 1). The coral community had changed so that corals that are more tolerant of stressful conditions (Porites spp. and soft corals) had replaced corals that are more sensitive to such conditions (Montipora spp.) (Darling et al. 2012).
References
Aronson RB, MacIntyre IG, Wapnick CM, O'Neill MW (2004) Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology 85:1876-1891
Baird AH, Guest J, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annual Review of Ecology, Evolution and Sytematics 40: 551-571
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Cote IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecology Letters 15:1378-1386
De'ath G, Moran PJ (1998) Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L.) on the Great Barrier Reef: 2: Feeding preferences. Journal of Experimental Marine Biology and Ecology 220:107-126
Graham NAJ, Nash KL, Kool JT (2011) Coral reef recovery dynamics in a changing world. Coral Reefs 30:283-294
Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Bijoux JP, Robinson J (2006) Dynamic fragility of oceanic coral reef ecosystems. Proceedings of the National Academy of Science, United States of America 103:8425-8429
Halford A, Cheal AJ, Ryan D, Williams DM (2004) Resilience to large-scale disturbance in coral and fish assemblages on the Great Barrier Reef. Ecology 85:1892-1905
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737-1742
Hughes TP, Tanner JE (2000) Recruitment failure, life histories and long-term decline of Caribbean corals. Ecology 81(8): 2250-2263
Johns KA, Osborne K, Logan M (2014) Contrasting rates of coral recovery and reassembly in coral communities on the Great Barrier Reef. Coral Reefs 33(3): 553-563
Nystrom M, Graham NAJ, Lokrantz J, Norstrom AV (2008) Capturing the cornerstones of coral reef resilience: linking theory to practice. Coral Reefs 27:795-809
Osborne K, Dolman AM, Burgess SC, Johns KA (2011) Disturbance and the Dynamics of Coral Cover on the Great Barrier Reef (1995-2009). PLoS ONE 6(3): e17516





