The Great Barrier Reef Today
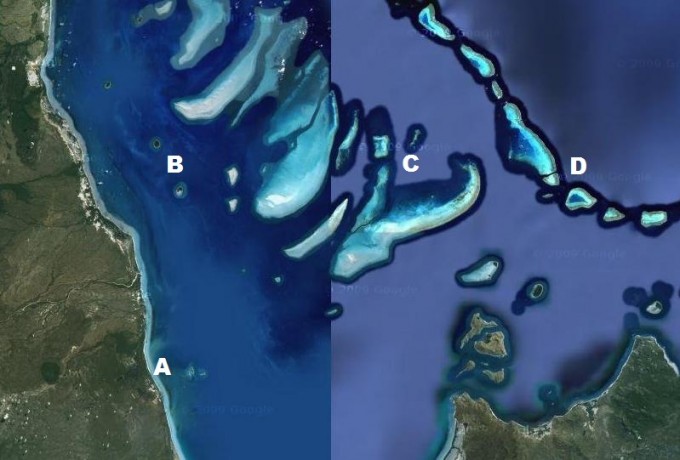
Extending over 2,000 km along the coast of Queensland, Australia and comprising some 2,900 individual coral reefs, the Great Barrier Reef [GBR] is the largest coral reef ecosystem to have existed in recent times. The northern most point of the GBR extends beyond Australian waters towards Papua New Guinea, whilst its southern most point terminates just north of Fraser Island off the south coast of Queensland. The width of the GBR varies along its length, extending from the low water mark on the Queensland coast past the edge of the continental shelf, and encompasses an entire area of approximately 344,400 square kilometres (133,000 sq mi), approximately the same size as Italy or Japan.
Life on the GBR
The GBR is a system composed of many highly diverse ecosystems that together house tens of thousands of species of plants and animals.The conservation of biodiversity on the GBR is an important challenge given the rich variety of plants and animals that are found here, including hundreds (in some cases thousands) of species of hard and soft corals, macroalgae, sponges, tunicates, bryozoans, jellyfish, polychaete worms, crustaceans, molluscs, starfishes and fishes. Some of these species are endemic (not found in any other geographic region in the world). Other species may become endemic to the GBR in the longer term, due to overexploitation or habitat degradation in other parts of their distribution, as in the case of the dugong, which has become extinct or near-extinct in many other parts of the world.
The GBR is also home to many species of marine mammals, seabirds and even a number of marine reptiles. It is estimated that up to 2000 species of fishes live on the GBR from the small reef fishes that characterise coral reefs, through solitary Coral Trout and hunting schools of Giant Trevally, to the graceful giant Whale Shark, which can exceed 10m (33ft) in length. Between 1.4 and 1.7 million seabirds live on the vegetated islands that dot the GBR and depend on the ocean for their diet. Marine mammals include the Dugong, and 26 species of dolphins and whales, such as the Dwarf Minke Whale. The marine reptiles found here comprise 6 sea turtle species, 17 sea snake species and one species of crocodile, the Saltwater or Estuarine Crocodile.
The GBR Today
The contemporary GBR is host to some of the most spectacular scenery on earth and is an area of outstanding natural beauty, receiving global recognition in 1981 when it was inscribed on the World Heritage List. The abundance and diversity of marine fauna and flora in the ocean is matched by the rich array of landscapes and seascapes ranging from densely vegetated islands, to teeming mangrove systems and the vast network of coral reefs. Reefs that have formed on the GBR continental shelf are known as barrier or shelf reefs and are distinct from deep ocean reefs such as atolls. They include fringing reefs, ribbon reefs and platform reefs. Fringing reefs form adjacent to the mainland or high islands. There are some 760 fringing reefs on the GBR. Platform reefs are oval in shape, typically 3 to 10 km long, and often have a lagoon in the middle. Some platform reefs accumulate sand in one section to form a coral cay, and this may be stable enough to support vegetation, and hence populations of birds and other small animals. Ribbon reefs occur only in the northern section of the GBR, close to the edge of the continental shelf. They are long and thin and lack a lagoon.
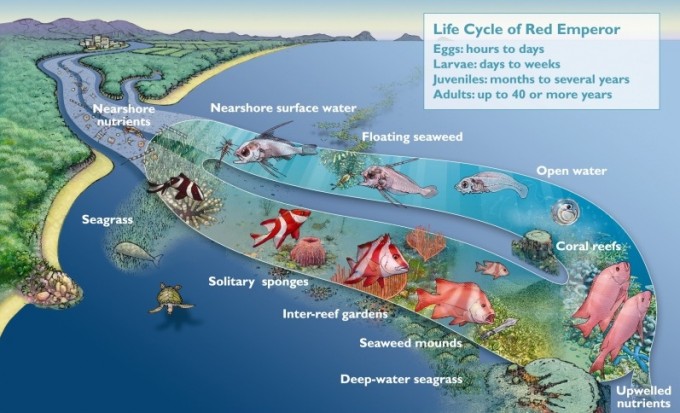
Despite the abundance of coral reefs on the GBR, they only account for approximately 6% of the habitats found here. Rocky reefs, sandflats, coastal mangroves, sponge gardens, seagrass beds and beaches are examples of other habitats that are present on the GBR, each of which play an important role in this intricate network of ecosystems (Fernandes et al. 2005). One example of this connectivity is found in the life cycle of the Red Emperor, which utilises a variety of different habitats and demonstrates the inter-connectedness of the different environments that make up the GBR.
Environment
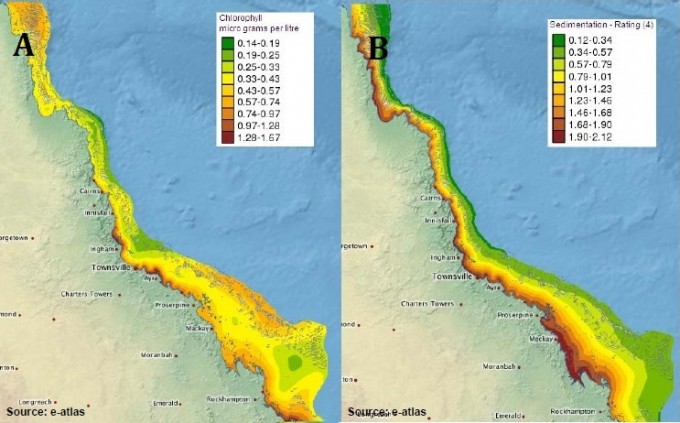
The geographic range encompassed by the GBR contains marked differences in environmental conditions along its length, breadth and depth. For example cyclone exposure varies between 5 per century in the far north and 20 per century in the southern GBR, while wave energy declines from the outer shelf to the Queensland coast, and with depth. The climate ranges from dry subtropical to wet tropical conditions (1000 to 5000 mm/yr precipitation), mean annual seawater temperature differs by ~3oC from north to south, and mean tidal amplitudes range from 1.5 to 7m. Water productivity, water clarity, chlorophyll concentration, and sedimentation vary more than fourfold along the coast-offshore gradient and with distance to major rivers (De’ath & Fabricius 2010). Along a latitudinal gradient (the length of the GBR) there is a net decline in species richness from north to south (Veron 1995; Veron and Stafford-Smith 2000; DeVantier et al. 2006; Fabricius and De'ath 2008), which is thought to result from the high energy supply and relatively low disturbance experienced in the northern GBR.
Habitat types also change strongly from the inner to outer-shelf of the GBR, which is partly explained by the variation in the physical environment across this gradient. Inner-shelf reefs receive strong terrigenous influences in the form of fresh water run-off which increase concentrations of nutrients and sediment, whilst the outer-shelf reefs are exposed to strong wave action and little particulate organic material (Newman and Williams 1996; De'ath and Fabricius 2010). In response, the composition of reef communities varies across the continental shelf, with the greatest diversity of coral communities being found at mid-shelf locations (Done 1982; DeVantier et al. 2006; Fabricius and De'ath 2008). There is also significant variation in the composition of fish assemblages both across the continental shelf and along the length of the GBR (Williams 1983).
Human Impact
Mankind is also active on the GBR. Our use of the GBR stretches back to the early history of the indigenous peoples of Australia for whom the GBR has deep cultural significance. Commercial and recreational fishing is common on the GBR, but in terms of revenue generated the tourism industry dwarfs fishing with an annual revenue upward of $5.1bn (McCook et al. 2010). Shipping lanes which intersect the GBR pose potential threats to the GBR through the introduction of foreign species in ballast water or on ship hulls. If ships run aground they are likely to cause severe habitat destruction especially if this leads to an oil spill, as with the Shen Neng 1 in April 2010. Human impact is not limited to marine activities, as coastal development and agriculture lead to heightened levels of nutrients and sediment, particularly on the inner-shelf of the GBR.
GBR Management
Effective management of the GBR is therefore critical in conserving the world’s largest reef system. In 2004 the Great Barrier Reef Marine Park Authority (GBRMPA) carried out a major review of zoning patterns on the GBR. Through consultation with relevant stakeholders a comprehensive revision of the Marine Park was carried out increasing ‘green zones’ (no-take zones protected from fishing and shipping) from just 4.6% to 33.3%. Also in recognition of the importance of each habitat on the GBR, 70 distinct bioregions (30 reef, 40 non-reef) were identified and at least 20% of each bioregion was protected under the new designation (Fernandes et al. 2005). Compliance however remains a challenge, with less than 0.3% of revenue generated from the GBR being spent on enforcement (McCook et al. 2010).
Collaboration between scientists and fishers is helping to expand understanding of the important roles that the marine reserves play in sustaining fish stocks and conserving biodiversity (McCook et al. 2010). This effort is supported by the extensive Long Term Monitoring Program; data on the health of the GBR that have been recorded by the Australian Institute of Marine Science (AIMS) since 1992. In the face of <climate change> and other threats such as <terrestrial run-off> and other forms of pollution, <cyclones>, outbreaks of crown-of-thorns starfish, <overfishing> and <coral diseases> the ecological health of the GBR will depend in the longer term on the responsibility of mankind to curtail our carbon emissions and minimise destructive practices.
References
De'ath G, Fabricius K (2010) Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecological Applications 20:840-850
DeVantier L, De’ath G, Turak E, Done T, Fabricius K (2006) Species richness and community structure of reef-building corals on the nearshore Great Barrier Reef. Coral Reefs 25:329-340
Done TJ (1982) Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1:95-107
Fabricius KE, De'ath G (2001) Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef reefs. Coral Reefs 19:303-309
Fabricius KE, De'ath G (2008) Photosynthetic symbionts and energy supply determine octocoral biodiversity in coral reefs. Ecology 89:3163-3173
Fernandes L, Day J, Lewis A, Slegers S, Kerrigan B, Breen D, Cameron D, Jago B, Hall J, Lowe D, Innes J, Tanzer J, Chadwick V, Thompson L, Gorman K, Simmons M, Barnett B, Sampson K, De'Ath G, Mapstone B, Marsh H, Possingham H, Ball I, Ward T, Dobbs K, Aumend J, Slater D, Stapleton K (2005) Establishing Representative No-Take Areas in the Great Barrier Reef: Large-Scale Implementation of Theory on Marine Protected Areas. Conservation Biology 19:1733-1744
Hopley D, Smithers SG, Parnell KE (2007) The Geomorphology of the Great Barrier Reef. Cambridge University Press, Cambridge
McCook LJ, Ayling T, Cappo M, Choat JH, Evans RD, De Freitas DM, Heupel M, Hughes TP, Jones GP, Mapstone B, Marsh H, Mills M, Molloy FJ, Pitcher CR, Pressey RL, Russ GR, Sutton S, Sweatman H, Tobin R, Wachenfeld DR, Williamson DH (2010) Marine Reserves Special Feature: Adaptive management of the Great Barrier Reef: A globally significant demonstration of the benefits of networks of marine reserves. Proceedings of the National Academy of Sciences
Newman SJ, Williams DM (1996) Variation in reef associated assemblages of the Lutjanidae and Lethrinidae at different distances offshore in the central Great Barrier Reef. Environmental Biology of Fishes 46:123-138
Renema W, Bellwood DR, Braga JC, Bromfield K, Hall R, Johnson KG, Lunt P, Meyer CP, McMonagle LB, Morley RJ, O'Dea A, Todd JA, Wesselingh FP, Wilson MEJ, Pandolfi JM (2008) Hopping Hotspots: Global Shifts in Marine Biodiversity. Science 321:654-657
Veron JEN (1995) Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. UNSW,Sydney
Veron JEN, Stafford-Smith MG (2000) Corals of the World. AIMS,Townsville
Williams DM (1983) Patterns in the distribution of fish communities across the central Great Barrier Reef. Coral Reefs 1:35-43