Patterns in hard coral diversity and cover on inshore reefs of the GBR

Identifying the principal spatial patterns in biodiversity of corals is a requirement for effective ecosystem management. This article summarises large-scale patterns in hard coral biodiversity on the GBR.
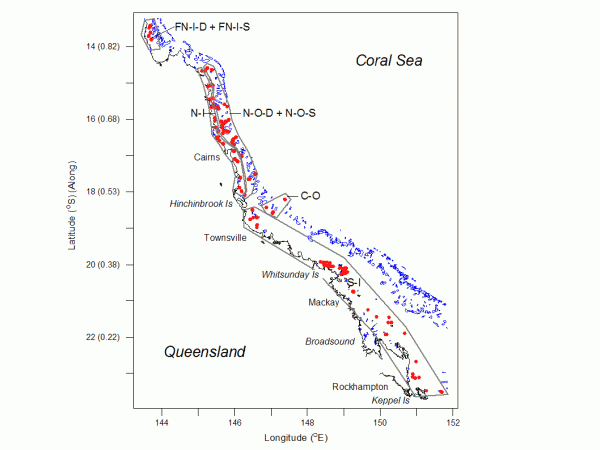
The study is based on one-off surveys of 599 sites on 118 inshore reefs and 17 mid-and outer-shelf reefs along 1300 km of Australia’s Great Barrier Reef (GBR) (Fig. 1), conducted between 1994 and 2001. More details are found in Devantier et al. (2006).
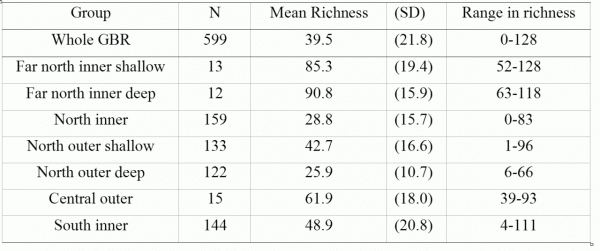
A total of 362 species from 75 genera in 15 families were recorded in this study. Combined with other studies, this brings the total hard coral record on the GBR to ~405 species in 78 genera. The far northern section of the GBR had highest mean and total site richness (ca. 86 and 130 spp. respectively). Ten new species were recorded in a five-day visit, suggesting more species remain to be discovered in the far north.
Coral species richness increased from the coast to the mid-continental shelf, and then declined towards the outer shelf. Diversity also increased with depth to five metres, then stabilised. The ‘Wet Tropics’ region and the Broad Sound – Northumberland Islands region have markedly reduced richness (67% and 50% compared with nearby regions). This difference is largely due, in the case of the Wet Tropics, to the cumulative impact of acute disturbances, including cyclones, predation by crown-of-thorns sea stars and river flood run-off, and in Broad Sound to marginal conditions for coral growth due to extreme tidal range and high turbidity slowing recovery from cyclones.
The study shows that on the GBR, disturbance results in the local removal of corals rather than a shift to other coral species. However, recent increases in impacts from river run-off, mass coral bleaching and crown-of-thorns sea star predation further threaten these communities, requiring continued, concerted management action.
Background
The GBR is formed of more than 3,000 individual reefs stretching ~2000 km and, with the adjoining inter-reefal area, covering 350,000 km2. About 80% of the reefs are platform reefs developed well offshore from the mainland coast on the mid- and outer-continental shelf, while about 600 reefs (20%; Hopley et al. 1989) are located nearshore, either as fringing reefs around continental islands and along the mainland coast, or as small detached platform reefs. The surveys reported here mainly focus on nearshore reefs and were based on standard Rapid Ecological Assessments.
Survey methods
All data were recorded by observers experienced in the field identification of reef-building corals (Devantier, Turak, Done) using SCUBA and snorkel. On each reef, up to three depth zones were surveyed, comprising (a) the shallow reef flat and crest; (b) the shallow reef slope (between 3 and 7 m depth); and (c) the deeper reef slope (maximum depth of 16 m). Only habitats considered suitable for development of coral-dominated communities were chosen. At each site, reef-building corals were identified to species level wherever possible (Veron 2000), otherwise to genus and growth form (e.g. massive Porites).
GBR coral species
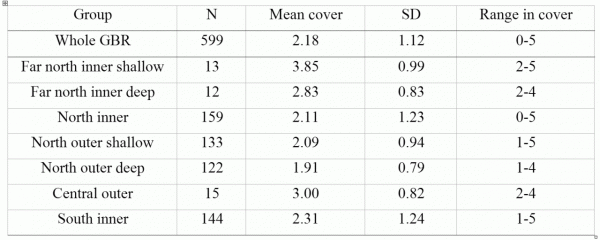
In total, 362 scleractinian (hard coral) species from 75 genera in 15 families were recorded at the 599 sites. Richness (the number of species of hard corals encountered at each site) averaged 39.5 species per site overall (range 0–128; Table 1). About 1.1% of sites had exceptionally high species richness (>100 species per site), ~30% of sites had moderate richness (>50 species), while ~20% of sites had low richness (<20 species). Together with the species not separated to species level in this study but also known to exist on the GBR (some encrusting Montipora and massive Porites, Veron 2000) the total record is ~405 coral species in 78 genera on the GBR.
Most of the 362 recorded species were rare or uncommon, occurring in only a small percentage of the sites surveyed. Eighty percent of species were recorded in less than 20% of the sites and 18% were recorded only 1–3 times, with 32 species recorded only once. Only 10% of species were moderately abundant, occurring in ≥30% of sites, and of these, only two taxa (Pocillopora damicornis and massive Porites spp.) were recorded in >80% of sites. Only eight species were found exclusively on mid- and outer-shelf reefs. Nine species were recorded only in the tropical waters of the far northern and northern reefs. Conversely, ~10 characteristic ‘sub-tropical’ GBR taxa occurred predominantly in the southern GBR (Devantier et al., 2006).
Patterns in species richness
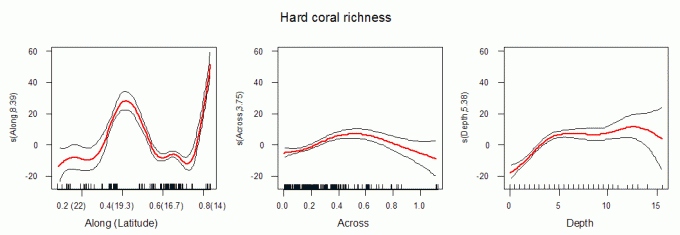
Richness changed more along the GBR than across the shelf or with depth (Fig. 2). Richness was highest in the far north (~14oS) and in the Townsville (Palm Islands) – Whitsunday Islands region (~20oS). The northern region (~15–18oS) showed a sharp decline in richness along a ~400 km long stretch. A second major decline in estimated site richness occurred south of 21oS (Northumberland Islands to Broad Sound), similar in magnitude to the northern trough.
Across the continental shelf, estimated site richness was ~10 species greater on mid-shelf sites than on either nearshore or outer-shelf sites (Figs 2 and 3). Richness was lower on the inner-most mainland fringing reefs and the outer-shelf platform and ribbon reefs and peaked in the middle section.
Species richness was highest at >5 m depth, remained near-constant to the deepest depths surveyed (~15 m), and declined linearly at shallower depths (<5 m), losing ~5 species m-1 (Fig. 2).
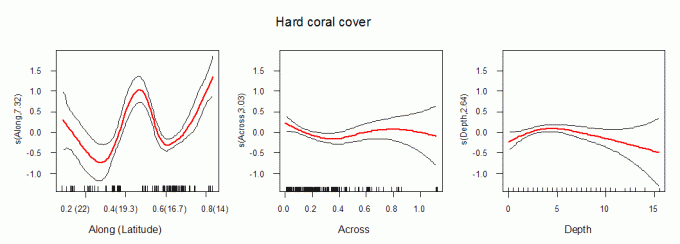
Patterns in hard coral cover
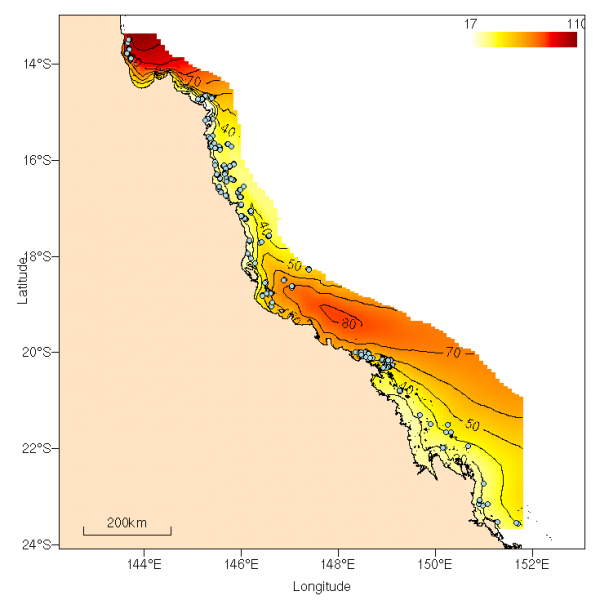
Hard coral cover varied with distance along the GBR, but was unrelated to relative distance across the GBR or depth (Fig. 4). Estimated cover was low in the northern region (~15–18oS) and in Broad Sound (~21–23oS), and highest in the far north and the Whitsunday Islands (Table 2). There was a weak positive relationship between richness and hard coral cover, which is not unexpected, given that on nearshore reefs, whole sites can be occupied by extensive beds of just a few species of genera such as Goniopora, Porites, Alveopora, Echinopora or Leptoseris. Conversely, other sites that were recovering from a past disturbance may be species rich, even at low levels of hard coral cover.
Patterns in coral communities
The analyses distinguished 12 spatially defined coral communities (Devantier et al., 2006). Most species were more abundant in the far northern inshore groups (both shallow and deep) and the offshore group, compared to the four remaining groups (Fig. 1). Importantly, none of the remaining northern and southern communities contained any indicator species. These poorer communities only contain species that also occur in higher numbers elsewhere.
Thirty-two species occurred across most sites. These species are generally ubiquitous and widely distributed beyond the GBR, and include species such as Pocillopora damicornis, Stylophora pistillata, Galaxea fascicularis and Acropora hyacinthus.
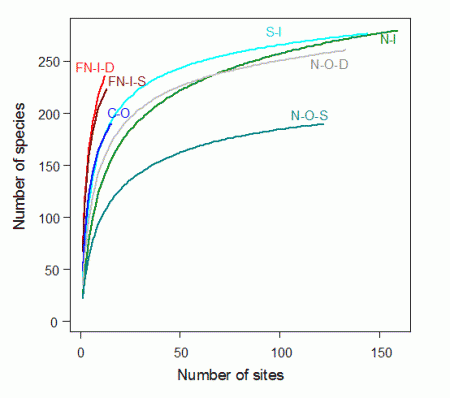
The far northern and central offshore communities were extraordinarily rich. Although they comprised less than 7% of all sites, they contained more than 85% of all species.
Cumulative species richness in these rich communities (labeled as FN-I-D, FN-I-S and CO in Fig. 5) had not begun to level off. The two far northern communities had the fastest rates of species accumulation, indicating that they are substantially richer than described here. In three of the other four communities, cumulative richness was of the order of c. 250 spp., with lower richness (c. 175 spp.) in the shallow northern offshore group (N-O-S, Fig. 5). This showed that the low coral richness in the other regions (northern inshore, northern offshore deep, and southern inshore (N-I, NO-D and S-I) were due to a low representation of taxa at individual sites, rather than to a small regional species pools.
What have we learned? – The GBR coral fauna
A total of 362 species in 75 genera of reef-building corals were recorded in the surveys. Together with the species not separated to species level in this study (some encrusting Montipora and massive Porites that are known to exist on the GBR (Veron 2000) the total species and generic richness of the GBR is ~405 species in 78 genera.
Most species present in the GBR were widely distributed across regions, but sparse in abundance across sites. Eighty per cent of species were recorded in less than 20% of the sites and 18% were recorded only 1–3 times. Just 8 of these 362 species were recorded only on mid- or outer-shelf reefs, confirming that a large majority of taxa can live (albeit some in low numbers) on nearshore reefs where water may be turbid from terrestrial influences and sediment re-suspension. Nine species were recorded only in the tropical far northern and northern waters (all being new records for the GBR); for these species the northern GBR appears to represent the margin of their distribution ranges (Veron 2000). In contrast, most of the 10 ‘sub-tropical’ GBR species found exclusively (in this study) in the southern and central GBR, also occur in sub-tropical or tropical regions elsewhere in the Indo-Pacific (Wallace 1999; Veron 2000). The reasons for their absence from the intensely surveyed more northern inshore areas of the GBR are unclear.
The taxonomic inventory in this study for the remote far northern communities is clearly an underestimate of total regional richness, as the species-abundance curves have not begun to plateau (Fig. 5).
Why and how do coral communities differ between regions?
The analyses distinguished 12 GBR communities. Although finer-scale structure was not presented in the broad large-scale analyses presented here, our best model suggests a separation of up to 22 communities, thus suggesting additional community types exist at finer spatial scales. As many coral communities share a substantial proportion of their coral species, a continuum of community structure exists over space and depth. Such continua in coral communities has also been reported elsewhere, e.g., in the Red Sea, and suggests that coral communities are more open than most communities in terrestrial systems.
Differences in species composition between inshore and offshore communities are partially explained by differences in the physiology of species. The inshore communities were characterised by a suite of species some of which are strong particle feeders well suited to turbid habitats where relatively high concentrations of fine suspended sediments and particulate matter provide food, but also affect illumination and potentially smother corals.
Other, more widespread species can switch from photosynthesis to particle feeding, maintaining a positive energy balance across a broad range of environmental conditions.
Characteristic species on offshore sites are mostly stout branching and massive corals including Acropora palmerae, Acropora abrotanoides, Acropora polystoma, Acropora multiacuta, Acropora monticulosa, Acropora lutkeni, Favia stelligera, Pocillopora verrucosa, Pocillopora eydouxi and Pocillopora woodjonesi. On the GBR, these species occur mostly in relatively clear waters, suggesting that their local populations are strongly photo-trophic.
Differences in ecological status between GBR regions
GBR coral communities have developed during the last 8000 years in an environment dominated by tropical storms and river floods. The intervals between extreme events may be important drivers of richness and cover. Disturbance regimes vary amongst regions; for example, there is a peak in cyclone activity between 20º and 21º latitude S (Massel and Done 1993), and there are shorter return periods between low salinity events in the depauperate Wet Tropics region than along other sectors of the inshore GBR. In the last 10 years, evidence has increased that increasing terrestrial runoff of fertilisers and silt from rivers onto the shallow and wide continental shelf is a significant problem for some nearshore reefs. There is also concern that fishing pressure, major population outbreaks of crown-of-thorns seastars Acanthaster planci, and mass coral bleaching have also increased in recent times. Most of the present study was undertaken prior to the 1998 and 2002 mass bleaching episodes, hence the apparent poor condition of the northern communities must be attributed to other causes.
The Far Northern communities are fantastically rich, and apparently developed in a regime of low recent disturbance. Few crown-of-thorns starfish are recorded in this region, water quality is little affected by land runoff, and fishing effort is lower than in the regions further south. The unsaturated species-abundance curves suggest that these regions are even richer than recorded here, and clearly more research should be conducted in this remote and highly diverse part of the GBR.
The Northern community also consists of a quite rich species pool, with more than 280 species recorded in total. Thus, low site richness scores in the Northern community are not due to a stunted regional species pool, but rather due to low abundances of many species at many sites, which were also found in other communities. This suggests that there has been no species replacement, i.e., no shifts in community composition from one species suite to another after disturbance. Rather, there was a generally low representation of a wide range of species. The sites likely represent degraded, yet potentially transitional, forms of the more diverse communities to their north and south (Figs. 1, 4; see also DeVantier et al. 1998).
The Northern Inshore community closest to the ‘Wet Tropics’ rivers (Community N-I) comprised of mostly widespread species in low abundance. This is consistent with the increasing evidence that these reefs are adversely affected by modified rivers discharging fine sediments, nutrients and pesticides, and several outbreaks of crown-of-thorns starfish.
The Northern Offshore communities (N-O-S and N-O-D) also had low site richness, low coral cover and also lacked strong indicator species at time of survey in 1995 – 1997. This was more than a decade after the previous highly destructive seastar outbreak series there and just prior to the next outbreak wave (Sweatman et al. 1998). It is possible that there had been insufficient time for establishment of the full potential richness and abundance at these sites. In contrast, the C-O had high richness at time of survey, despite similar recovery time from the 1980s seastar outbreaks. At C-O, recovery was well underway during the surveys (however most of these and the northern reefs have subsequently been impacted again by the seastars).
The Southern community is a large region which includes the species-rich Palm Island and Whitsundays regions, as well as the poorer Broadsound and Keppel regions. More fine-scaled analyses confirm a greater separation in communities than presented here (e.g., Van Woesik and Done 1997; and Devantier et al., 1998). The shallow and turbid Broad Sound region with its up to 7 m tidal range is known to be naturally marginal for coral growth and reef development (Kleypas 1996). Coral richness and cover increases with increasing distance offshore from Broad Sound and northwards to the Northumberland Group (van Woesik and Done 1997). Further south away from Broad Sound, low cover and richness at many Northumberland and Keppel sites at the time of survey (1997) were a legacy from the major coral mortality caused by the flooding Fitzroy River in 1990, and a limited time for recovery. Again, communities were characterised by a lack of species, rather than replacement by another suite of species between disturbances.
During the time of the surveys, the state of GBR coral communities was considered quite unaffected by human activity. Since most of this study was undertaken, many GBR reefs have been affected by mass bleaching events in 1998 and 2002, and many have been subjected to the third destructive outbreak of A. planci since the 1960s. With likely synergistic effects of future mass bleaching episodes, A. planci outbreaks and river run-off, the capacity for recovery of the inshore reefs may be severely put to the test. It is conceivable that the diminished characteristics of the northern communities will become more widespread, through a region-wide depletion and loss of less tolerant, more specialized taxa and loss of their reproductive outputs. Such changes would represent a severe reduction in ecological complexity and diversity. It would likely cascade from reef to reef through weakening supply of larvae of many species of reef inhabiting organisms, and cascade from corals to other fauna (Jones et al. 2004). It is hoped that a network of no-take zones across the length and breadth of the GBR [link to GBRMPA rep area site] would provide the presently best option to safeguard against such undesirable changes.
References
DeVantier L, De'ath G, Done T, Turak E, Fabricius K. 2006. Species richness and community structure of reef-building corals on the nearshore Great Barrier Reef. Coral Reefs 25: 329-340
Devantier LM, De'ath G, Done TJ, Turak E. 1998. Ecological assessment of a complex natural system: A case study from the Great Barrier Reef. Ecological Applications 8: 480-496
Done TJ. 1982. Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1: 95-107
Hopley D, Parnell K, Isdale P. 1989. The Great Barrier Reef Marine Park: Dimensions and regional patterns. Australian Geographical Studies 27: 47-66
Kleypas JA. 1996. Coral reef development under naturally turbid conditions: fringing reefs near Broad Sound, Australia. Coral Reefs 15: 153-167
Massel SR, Done TJ. 1993. Effects of cyclone waves on massive coral assemblages on the Great Barrier Reef: Meteorology, hydrodynamics and demography. Coral Reefs 12: 153-166
R Development Core Team. 2008. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL https://www.r-project.org.
Van Woesik R, Done TJ. 1997. Coral communities and reef growth in the southern Great Barrier Reef. Coral Reefs 16: 103-115 Veron J. 2000. Corals of the World. Australian Institute of Marine Science, Townsville
Veron JEN. 1995. Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. University of New South Wales Press, Sydney
Wallace C. 1999. Staghorn corals of the World. CSIRO Publishing, Collingwood
Wood S. 2000. Modelling and smoothing parameter estimation with multiple quadratic penalties. Journal of the Royal Statistical Society Series B 62: 413-428