Juvenile corals on the Great Barrier Reef - the sex life of corals and the importance of recruitment to coral reef recovery.

A brief history about the sex life of corals
Corals have an interesting life cycle and spend part of their lives floating around in the sea and part of their lives stuck to the reef. Adult corals are actually colonies made up of many organisms called polyps. Within a colony polyps can be male or female (gonochoristic) or both (hermaphroditic) (Baird et al 2009). There are two ways corals reproduce. Adult coral colonies can release their sperm and eggs (gametes) into the water column (spawning), where the eggs are fertilised. This embryo develops into a planula larva (sometimes it is called a planula or larva). Other corals have some maternal protection, as they retain or brood larvae within the colony and release them later once they are more developed (Baird et al 2009). Some coral species spawn and brood, but this is quite rare (Baird et al 2009). During summer many corals spawn at certain times of the month, for several months, but this can vary between species (Harrison et al 1984, Willis et al 1985, Baird et al 2009). These larvae are capable of swimming, but they are very small and are usually at the whims of the tides and currents. The larvae drift around in the water column and slowly develop the ability to sense surroundings. After some time drifting around in the ocean, a larva will develop the ability or competency to settle on the substrate.
How corals come to lead a life on the reef
In order for larva to settle they usually need a reason. Scientists call this a cue. Larvae respond to cues associated with coralline algae (Heyward and Negri 1999, Negri et al 2001). Known cues include specific chemicals from bacteria that live on some species of coralline algae. For example, tetrabromopyrrole is a metabolite that was extracted at AIMS from a bacteria, Pseudoalteromonas sp., associated with the coralline algae Neogoniolithon fosliei (Siboni et al 2014). Once larvae encounter a cue they can settle onto the reef base, otherwise they can spend many months floating at sea (Graham et al 2008). Once a larva settles, it secretes a calcium carbonate skeleton, attaching it to the reef base. This transition from a passive life in the water column to a sedentary life on the reef framework is known as metamorphosis. If a newly metamorphosed coral doesn’t like where it’s living, it can leave its newly secreted skeleton, a process called reverse metamorphosis (Richmond, 1985). A larva can settle again, but since most coral larvae don’t feed they can only do this a limited amount of time, since these processes use a lot of energy.
After settlement and metamorphosis corals are very small (0.32-2.7mm) and their size depends on the species (Babcock et al 2003, Baird and Babcock 2000). Despite much research, there is still little information on the very early life histories of some coral genera, because the newly settled corals are difficult to see and count. To overcome this several teams have used fluorescent lights to help spot them in underwater surveys, although due to their small size it is difficult to get more information than abundance and their size (Roth and Knowlton 2009). For more detailed information on the development of newly settled corals, scientists have to examine them under microscopes in the laboratory (Babcock et al 2003).
To help corals obtain energy, most have tiny algae called zooxanthellae that live within the coral's tissue and make corals appear brown. Zooxanthellae can be considered the solar panels of a coral, as they fix energy from the sun and make it available to the corals for growth and development. It is still unclear when zooxanthellae start the symbiosis within the coral tissue. Current knowledge suggests some obtain these through their mothers, but most coral species uptake zooxanthellae in the environment and can do so before they settle (Cumbo et al 2013).
Recently metamorphosed corals still face many threats, including getting eaten by fish, getting overgrown by the neighbouring organisms and getting smothered by sediment (Penin et al 2010, Trapon et al 2013). Studies have shown that after coral settlement, corals face a tough time, and many die during the first few months after they start life on the reef (Babcock 1985, Babcock and Mundy 1996, Cooper et al 2014, Penin et al 2010). As corals grow, they become easier to see without the use of fluorescent lights. This makes it easier for scientists to count the corals while diving. Most corals less than 5cm in diameter are not able to reproduce, so are often called juvenile corals.
Coral juveniles and reef recovery
The long-term monitoring program (LTMP) at the Australian Institute of Marine Science introduced juvenile coral surveys to its extensive coral reef monitoring program in 2007 (Figure 1) (Jonker et al 2008). Our team now have 7 years of data that is being analysed and written up by scientists.
Figure 1. The location of survey reefs where AIMS LTMP collects data. Surveys of juvenile corals are recorded at a subset of reefs.
Juvenile corals can be used as a measure of coral replenishment. For example, the data from a 50m transect at North Reef on the Southern GBR shows an initial increase in coral juveniles followed by an increase in hard coral cover two years later (Figure 2).
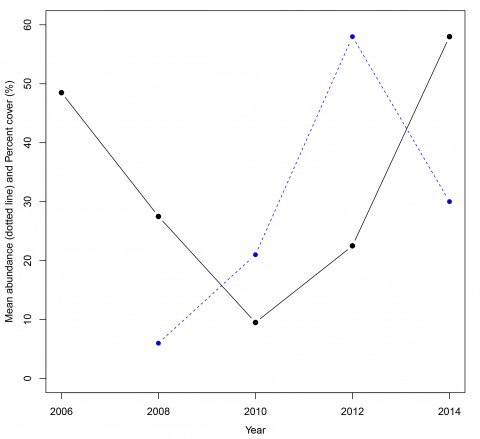
At the start of surveys in 2006, coral cover was high and dominated by Acropora branching and plate corals (Figure 2).

After storms and cyclones in 2008 and 2009 there was low structural complexity and low coral cover (Figure 3). Through recruitment of corals and their growth we started to see coral recovery in the juveniles in 2010 followed by hard coral cover in 2012. In 2014 coral cover on this small section of reef had reached pre-disturbance levels and based on regional data is expected to increase further.
In the example above, coral cover is increasing, but does this equate to reef recovery? The recovery of coral cover does not necessarily mean the reef will look the same or serve the same functions for the animals that use corals for habitat or food. Reassembly is a term used to compare whether or not the composition of a reef returns to the ratios of coral genera and growth forms prior to disturbance (Johns et al 2014). Coral recovery is typically quicker than reassembly (Johns et al 2014) and in this time series there are fewer branching corals in 2014 than in the pre-disturbance community.
References
Babcock R (1985) Growth and mortality in juvenile corals (Goniastrea,Platygyra and Acropora): the first year. In: Proceedings of the 5th international coral reef congress 4:355–360
Babcock R, Mundy C (1996) Coral recruitment: consequences of settlement choice for early growth and survivorship in two scleractinians. J Exp Mar Biol Ecol 206:179–201
Babcock RC, Baird AH, Piromvaragorn S, Thomson DP, Willis BL (2003) Identification of scleractinian coral recruits from Indo-Pacific reefs. Zoological Studies 42:211-226
Baird AH, Babcock RC (2000) Morphological differences among three species of newly settled pocilloporid coral recruits. Coral Reefs 19:179-183.
Baird AH, Guest JR, Willis BL (2009) Systematic and Biogeographical Patterns in the Reproductive Biology of Scleractinian Corals. Annual Review of Ecology, Evolution and Systematics. doi:10.1146/annurev.ecolsys.110308.120220
Cooper W, Lirman D, Porter VanGroningen Parkinson JE, Herlan J, McManus JW (2014) Assessing techniques to enhance early post-settlement survival in situ for reef restoration. Bulletin of Marine Science. 90: 651-664.
Cumbo VR, Baird AH, van Oppen MJH (2013) The promiscuous larvae: flexibility in the establishment of symbiosis in corals. Coral Reefs. 32:111-120.
Emslie MJ, Cheal AJ, Johns KA (2014) Retention of Habitat Complexity Minimizes Disassembly of Reef Fish Communities following Disturbance: A Large- Scale Natural Experiment. PLoS ONE 9(8): e105384. doi:10.1371/journal.pone.0105384
Graham EM, Baird AH, Connolly SR (2008) Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27: 529-539
Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL (1984) Mass spawning in tropical reef corals. Science 223: 1186-1189.
Heyward AJ, Negri AP (1999) Natural inducers of coral larval metamorphosis. Coral Reefs 18:273-279.
Johns KA, Osborne KO, Logan M (2014) Contrasting rates of coral recovery and reassembly in coral communities on the Great Barrier Reef. Coral Reefs 33: 553-563. doi:10.1007/s00338-014-1148-z
Jonker M, Johns K and Osborne K (2008) Surveys of benthic reef communities using underwater digital photography and counts of juvenile corals. Long-term Monitoring of the Great Barrier Reef. Standard Operational Procedure. No. 10. Australian Institute of Marine Science.
Negri AP Webster NS Hill RT Heyward AJ (2001) Metamorphosis of broadcast spawning corals in response to bacteria isolated from crustose algae. Marine Ecology Progress Series 223:121-131.
Penin L, Michonneau F, Baird AH, Connolly SR, Pratchett MS, Kayal M, Adjeroud M (2010) Early post-settlement mortality and the structure of coral assemblages. Marine Ecology Progress Series 408:55-64.
Richmond RH (1985) Reversible metamorphosis in coral planula larvae. Marine Ecology Progress Series. 22: 181-185.
Roth MS, Knowlton N (2009) Distribution, abundance and microhabitat characterization of small juvenile corals at Palmyra Atoll. Marine Ecology Progress Series. 376: 133-142. doi: 10.3354/meps07787
Siboni N, Abrego D, Motti C, Tebben J, Harder T (2014) Using bacterial extract along with differential gene expression in Acropora millepora larvae to decouple the processes of attachment and metamorphosis. PLoS ONE 9: e91082 doi:10.1371/journal.pone.0091082
Trapon ML, Pratchett MS, Hoey AS, Baird AH (2013) Influence of fish grazing and sedimentation on the early post-settlement survival of the tabular coral Acropora cytherea. Coral Reefs 32:1051-1059.
Willis BL, Babcock RC, Harrison PL, Oliver JK, Wallace CC. (1985) Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. In: Proceedings of the Fifth International Coral Reef Congress, pp. 343-348. From: Fifth International Coral Reef Congress, 27 May - 1 June 1985, Tahiti, French Polynesia.





